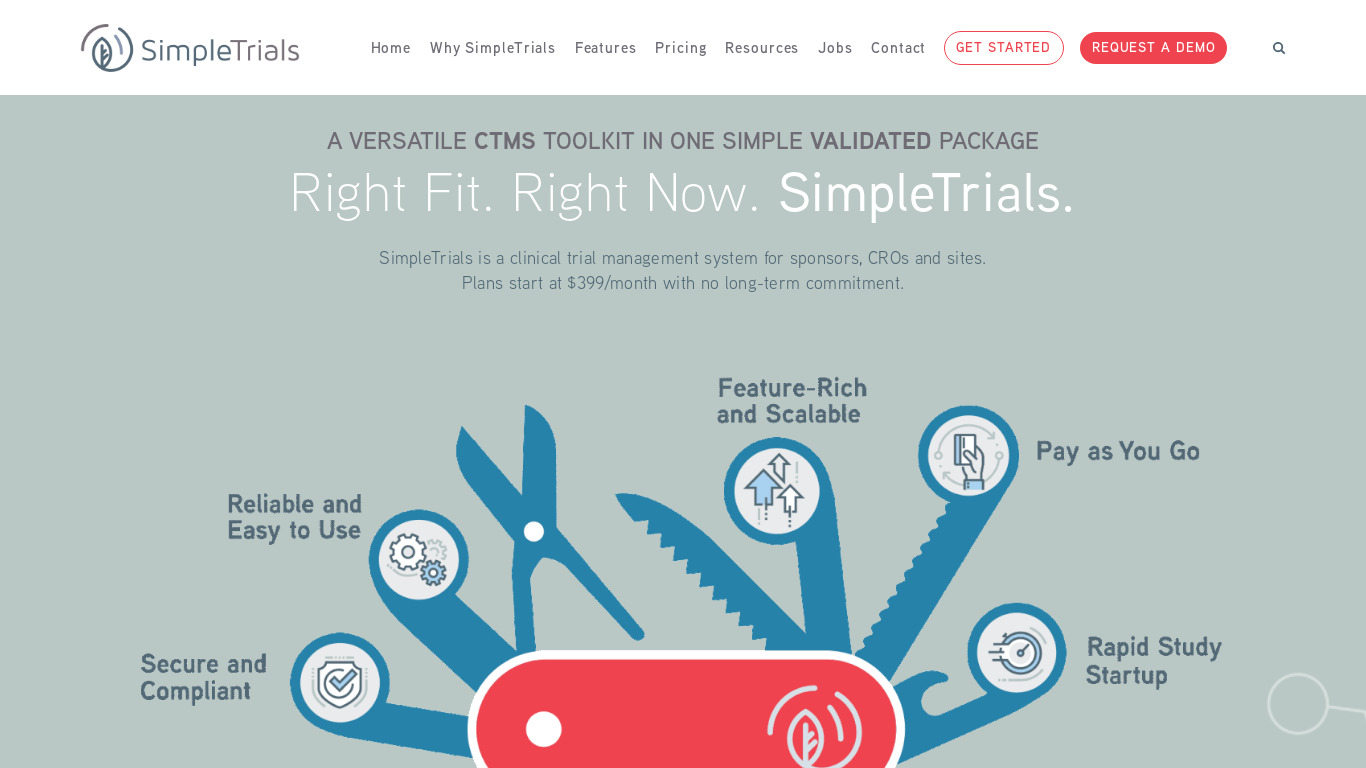
SimpleTrials VS Protocol Manager (CTMS)
Compare SimpleTrials VS Protocol Manager (CTMS) and see what are their differences

Consent-Based Identification of the Person Visiting Your Website Including First Name, Last Name, Email & 37 Other Data Points. Identify and Influence Your Engaged Website Visitors into Sales-Ready Leads – Before You Commit a Single Working Hour.
featured