

 1factory
1factory
 Qualio
Qualio
 EtQ Reliance
EtQ Reliance
 Propel
Propel
 COMPASS Quality Management System
COMPASS Quality Management System
 IQMS ERP
IQMS ERP
 TrackWise
TrackWise
 Ease
Ease
 Essenvia
Essenvia
 Greenlight Guru
Greenlight Guru
 QIT Enterprise Quality Management
QIT Enterprise Quality Management
 FORM.com
FORM.com
 nessViewer
nessViewer
 Arena QMS
Arena QMS
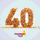 Pharma Test Galenic Instruments
Pharma Test Galenic Instruments
 NZBGet
NZBGet
1factory currently serves over 100 product manufacturers across Aerospace, Medical Device, Semiconductor Equipment, Automotive, Precision Optics, Consumer & Pharmaceutical Packaging, and Industrial Equipment markets.
Features include: 1. PDF Drawing Ballooning, First Article Inspection, AS9102 2. Quality Control Plans 3. Incoming, In-Process, Finished Goods Inspections 4. Digital Gage, CMM, and Test Fixture Data Import 5. Process Capability (Cp, Cpk, Pp, Ppk) 6. SPC, Run Charts, Histograms 7. Traceability: Batch Number, Serial Number, Gage, Inspector 8. Factory Defect Risk Monitoring 9. Non-Conformances, Corrective Actions, Audit Findings 10. Gage Calibration and Tracking 11. Supplier Collaboration & Real-Time Data Sharing 12. Supplier Management & Scorecards
Essenvia is an online platform to streamline regulatory pathway for medical device companies. FDA research data indicates that regulatory submissions have grown in complexity and size with an average of 1185 pages per submission, with a consistent 30% rejected at initial review and 64% sent back for additional information. Essenvia’s regulatory software streamlines the regulatory submission process by improving collaboration and automating some of the most time-consuming and error-prone tasks. Submissions processed on Essenvia reduce time and effort by 40% and eliminate 52% of errors. We are determined to reduce avoidable errors in regulatory submission and help medical device companies accelerate time to market.
 1factory
1factory Essenvia
EssenviaNo features have been listed yet.
Qualio - Qualio is a web based quality management platform that simplifies compliance for small to mid sized life sciences companies.
Greenlight Guru - QMS Software for Medical Device Companies | Quality Management Software
EtQ Reliance - QMS integrates data to reduce risk and ensure compliance.
QIT Enterprise Quality Management - QIT Quality Management system (QMS) contains CAPA, SCAR, Complaints, Nonconformance/NCR, Supply Chain, Audit, Engineering Change/Deviation Control and more
Propel - Propel is a customer-centric PLM software built on Salesforce App Cloud platform.
FORM.com - FORM.com is an enterprise mobile forms software and solutions perfect for optimizing online and offline inspection, audit, compliance operations and more.